Patient Centered Vestibular Schwannoma Management: Patient & Public Involvement (PPI) group - June 2023 Group Meeting
Recently, we organized a Public and Patient Involvement (PPI) group with Vestibular Schwannoma patients to understand their perspectives on an patient-centered automated report. Partnering with the British Acoustic Neuroma Association (BANA), we recruited participants by circulating a form within the BANA community through their social media platforms.
This approach garnered interest from a wide pool of participants across the UK, with over 200 VS patients expressing their willingness to take part. From this group, 12 individuals were thoughtfully selected to ensure diverse representation in terms of age, gender, and treatment paths, thus enriching the workshop’s insights. Further, to ensure broad participation from patients across the UK, this event was conducted online.
During the patient involvement event, Jonathan Shapey, consultant neurosurgeon, commenced the session by providing a clinical context on Vestibular Schwannoma and discussing the potential implications of automation in clinical care. Subsequently, Navodini Wijethilake presented a concise overview, outlining the automated report generation process and the pivotal role that Artificial Intelligence plays in addressing the clinical challenges.
The workshop then transitioned into small focus group discussions, each led by the impartial facilitator (William Hunter), Jonathan, and Navodini. These groups, comprising of 4 participants, facilitated candid conversations around several pertinent themes. We explored participants’ prior knowledge of the process, their feelings and concerns about automated measurements, and their preferences regarding visual aids for tracking tumour progression.
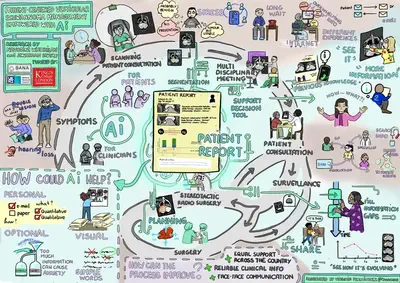
The workshop ended with a large group discussion that brought together the main ideas from the focus group sessions. An impartial facilitator guided the workshop, and an artist captured visual notes of the day’s activities and discussion points. This event was funded by the MRC DTP Flexible Supplement Fund.