A prospective observational study to evaluate the use of an intraoperative hyperspectral imaging system in neurosurgery.
IRAS: 284230. REC reference: 22/LO/0046.
ISRCTN Identifier: ISRCTN23639281
ClinicalTrials.gov Identifier: NCT05294185
Award ID: NIHR202114
Contact the NeuroHSI team using our contact form.
The study
A study to demonstrate that a novel, light based, imaging system could be used in neurosurgical procedures in the future with a view to improving outcomes
The key points
- Will not affect your operation or outcome
- Study to assess whether the system is capable of obtaining helpful images for surgical guidance in the future
- May add a maximum of 15 minutes to your operation time in order to obtain these images but no other aspect of the procedure will be carried out any differently
- No radiation, the system uses safe light only
- No additional follow-up visits or contact required

More details can be read in our Plain English Summary and Scientific Abstract.
Patient and public involvment
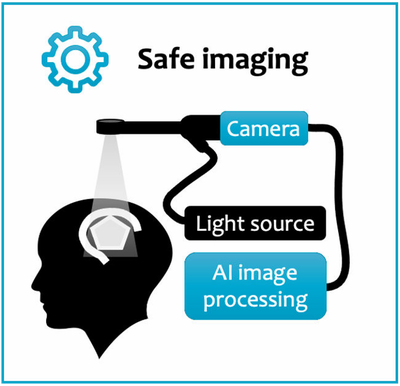
Hyperspectral imaging systems allow us to interpret bands of light beyond what the naked eye can see. It has already been used to great effect in other industries and has begun to show some promise within clinical practice. Our novel system employs this technology into the neurosurgical theatre with the expectation that it will be able to provide valuable, live information to the surgeon allowing them to carry out safer operations for our patients.
The purpose of this study is to assess the quality of data obtained by our system and how well it integrates into current neurosurgical practice. We are currently running a public engagement group that had its first meeting on 9th February 2022, providing valuable to our research team on this study.
We plan to meet 6 monthly to annually. If this is something you would like to be involved in please use the contact form. Similarly, if you would like more information about what the study entails from a patient perspective, please take a look at our study patient group pamphlet and our patient information sheets (PIS):
Alternatively, feel free to contact us and one of the research team will get back to you.
News
On 21st September we held our fourth ‘Science for Tomorrow’s Neurosurgery’ PPI group meeting online, with presentations from Oscar, Matt and Silvère. Presentations focused on an update from the NeuroHSI trial, with clear demonstration of improvements in resolution of the HSI images we are now able to acquire; this prompted real praise from our patient representatives, which is extremely reassuring for the trial going forward. We also took this opportunity to announce the completion of the first phase of NeuroPPEYE, in which we aim to use HSI to quantify tumour fluorescence beyond that which the human eye can see. Discussions were centered around the theme of “what is an acceptable number of participants for proof of concept studies,” generating very interesting points of view that ultimately concluded that there was no “hard number” from the patient perspective, as long as a thorough assessment of the technology had been carried out. This is extremely helpful in how we progress with the trials, particularly NeuroPPEYE, which will begin recruitment for its second phase shortly. Once again, the themes and discussions were summarized in picture format by our phenomenal illustrator, Jenny Leonard (see below) and we are already making plans for our next meeting in February 2024!
Plain English Summary of the NeuroHSI research project
Brain surgery operations include brain tumour removal and blood vessel procedures. Each year in the UK, approximately 70,500 patients are diagnosed with a brain tumour, 5,000 of whom undergo surgery. Approximately 1,000 patients undergo blood vessel brain surgery. Brain tumour surgery involves removing as much of the tumour as safely as possible. If all tumour is removed, patients have significantly better outcomes and live longer.
However, even with the best hands and the most modern technology currently available, it is often not possible to reliably identify tumour during surgery. Moreover, nerves and blood vessels cannot be reliably identified either during surgery. Yet, they need to be preserved to avoid brain damage. Due to this uncertainty and the need to balance risks, tumour is often left behind. Today, close to 30% of brain tumour patients require repeat surgery owing to tumour left behind during their first surgery. Further surgeries are more difficult, pose additional patient risks and lead to increased healthcare costs with often poor patient outcomes.
Newly developed camera systems have the potential to enhance the surgeon s vision to reliably identify tumour and healthy brain structures. Hyperspectral imaging (HSI) is one of the most promising of such technologies. Its core ability is to provide very detailed and rich information that is invisible to the human eye. HSI has demonstrated the potential to provide crucial, but currently unavailable, information about tumour and critical brain structures during surgery. However, HSI data is very complex and requires advanced computer-processing for its interpretation. In two previous clinical studies, we have shown that we can safely integrate our HSI prototype system during surgery.
In this project, we will further advance our prototype, refine our setup and develop key computer-processing features. We will assess our system in a new clinical study involving 81 patients undergoing brain tumour and blood vessel surgery. The outcomes of this study will inform the commercialisation of the technology for patient and NHS benefits.
This study has been designed with patient input and feedback from various patient groups including The Brain Tumour Charity. We received feedback stating that this research in improving surgical outcomes is potentially life-changing, drastically improving patient health, wellbeing and quality of life. The study will form a Patient Advisory Group to advise on all stages of the research. A patient representative, named public co-applicant on the project team, will sit on the study management board. We will ensure that the patient voice is expressed clearly in study reports and when sharing research updates more widely. Results will be made publicly available through publication of open-access research papers, presentation of results at scientific meetings, patient events, and online via King’s College London and relevant charity websites/social media.
Scientific Abstract
To address the pressing clinical need of improved surgical precision and patient safety during neurosurgery, we will develop a medical device capable of contact-free, contrast-agent-free, wide-field imaging and real-time tissue characterisation for seamless surgical guidance. Our innovation relies on advancing data-driven hyperspectral imaging (HSI).
Even though the ability of HSI to differentiate tissue types and measure tissue characteristics is supported by a relatively large body of scientific evidence, to date, commercial HSI systems are used mainly for tissue healing analysis rather than for surgical guidance. Indeed, existing systems are slow (20s per image) and lack sterility features vital for surgical guidance.
This project builds on our previous research with two successful first-in-patient studies demonstrating that our initial intraoperative HSI (iHSI) prototype seamlessly integrates into the surgical workflow. To deliver on this primary objective, our project will pursue eight aims for our iHSI technology:
- Demonstrate clinical safety in neurosurgery
- Establish correlation with histology
- Develop quantitative perfusion algorithms
- Achieve clinical-grade wide-field perfusion estimations
- Demonstrate accurate differentiation of tissue types
- Advance regulatory compliance towards commercialisation
- Dissemination of the research to patients and the public
- Protect innovation and disseminate findings to the research community
Our project plan, executed within a 36-month period, is divided into five work packages (WPs) spanning:
- Clinical implementation (centred on a prospective clinical study involving 81 patients)
- iHSI hardware design and system characterisation
- Real-time computational algorithms design
- Product development following MDR/UKCA-requirements
- Management, PPI and dissemination
The close collaboration between King’s College London, King’s College Hospital and Hypervision Surgical Ltd (King’s spin-out) will ensure a fast-tracked conversion from a healthcare innovation into a product achieving accelerated patient and public benefit. By the end of the project, our technology will be ready for MHRA submission and for inclusion in prospective clinical efficacy studies.
Contact
NeuroHSI team
- 1 Lambeth Palace Road, London, SE1 7EU, UK
- Becket House 9th floor